Question: What is the Right to Try Act?
Answer: The Right to Try Act is a law that was passed by unanimous consent in the Senate, a large bipartisan majority, 267-149, in the House, and signed by President Trump in May 2018. The law permits patients with life threatening conditions the right to use investigational drugs as long as the drug has completed Phase 1 clinical trials and remains under investigation. The aim of the legislation is to allow dying patients access to investigational drugs they would otherwise not be able to access.
On the state level, Right to Try laws are already in place in 41 states and counting: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, South Carolina, South Dakota, Tennessee, Texas, Utah, Virginia, Washington, West Virginia, Wisconsin, and Wyoming.
Question: Who qualifies under the Right to Try Act?
Answer: To be eligible under the Right to Try Act, a patient must meet the following conditions:
- Be diagnosed with a life-threatening disease or condition
- Have exhausted approved treatment options
- Be unable to participate in a clinical trial involving the eligible investigational drug, as certified by a doctor, who is in good standing with her licensing organization and will not be compensated directly by the manufacturer for so certifying; and
- Give written informed consent regarding the risks associated with taking the investigational treatment.
Question: What is a life-threatening disease or condition?
Answer: Federal law defines a life-threatening disease or condition as: “Diseases or conditions where the likelihood of death is high unless the course of the disease is interrupted” (21 CFR 312.81).
Question: What drugs or treatments qualify for Right to Try?
Answer: The treatments available under the law must meet the following conditions:
- Have completed an FDA-approved Phase 1 clinical trial;
- Be in an active clinical trial intended to form the basis of an application for approval or be the subject of an application for approval that has been filed with the FDA; and
- Be in ongoing active development or production and not discontinued by the manufacturer or placed on clinical hold.
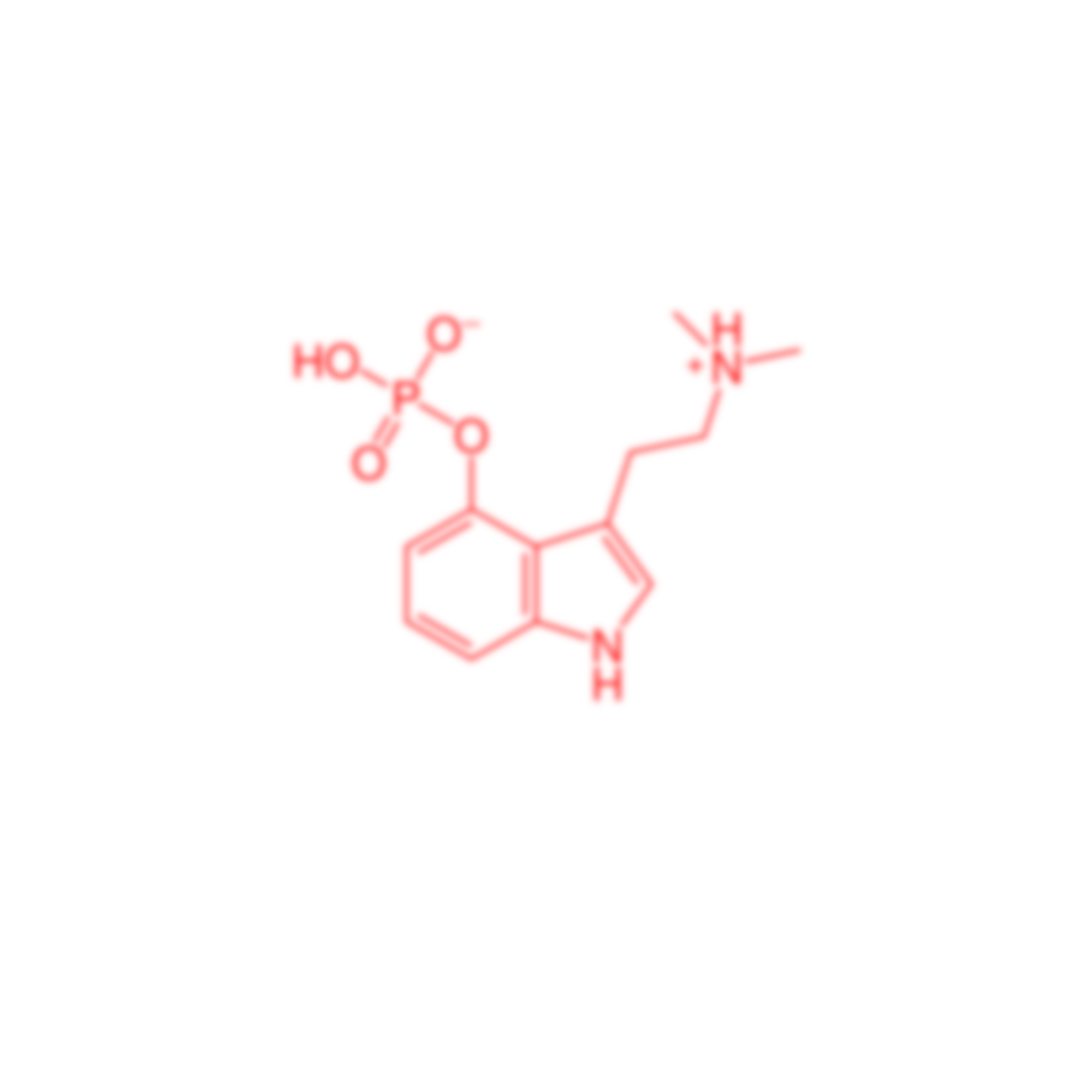
Question: What is psilocybin?
Answer: Psilocybin (sy-lə-SY-bin) is a naturally occurring psychedelic, prodrug compound produced by more than 200 species of fungi. Laboratory-produced psilocybin has completed Phase 1 and Phase 2 clinical trials and remains under investigation. Psilocybin was designated a “breakthrough therapy” by the FDA and shows promise to be an approved drug when clinical trials are complete.
Question: Why should terminally ill individuals have the Right to Try Psilocybin?
Answer: The Right to Try Psilocybin (RTTP) should be available to all dying patients. Extensive research has shown that psilocybin can bring “immediate, substantial, and sustained improvements in anxiety and depression; decreased demoralization and hopelessness, improved spiritual well-being, increased quality of life.“
Question: Why did advocates engage in civil disobedience at the Drug Enforcement Administration HQ?
Answer: The Drug Enforcement Administration (DEA) is preventing terminally ill patients from legally accessing psilocybin. The administration incorrectly asserts that the Controlled Substances Act, which scheduled psilocybin in the most restrictive category, prevents dying patients from accessing the drug even though it qualifies under the Right to Try Act. RTTP advocates have submitted both a petition to reschedule psilocybin off of Schedule I on Feb. 2, 2022 and a request the agency grant a waiver for RTT access on Feb 10. The DEA has not responded to either the petition or the request. RTTP advocates are demanding the DEA stop their delays and believe the best way to make the agency comply is come to their front door.
Question: Who else supports the Right to Try Psilocybin?
Answer: In the winter of 2022, U.S. Representatives Earl Blumenauer (D, Oregon), Rashida Tlaib (D, Michigan), Don Bacon (R, Nebraska), Dean Phillips (D, Minnesota), Madeleine Dean (D, Pennsylvania) Sheila Jackson Lee (D, Texas), and Andy Biggs (R, Arizona), who serves as chairman of the House Freedom Caucus, all signed on to a letter to the DEA asking the agency to allow RTT access to psilocybin. In 2021, the Attorneys General from Washington, Arizona, Delaware, Illinois, Michigan, Minnesota, Ohio, Oregon, and the District of Columbia signed on to an amicus brief in support of RTT access.
Some of these questions and answers were adapted from the Goldwater Institute’s RightToTry.org website. The Goldwater Institute has worked tirelessly to pass RTT legislation across the United States and deserves full credit for their effort to help dying citizens.