FOR IMMEDIATE RELEASE
Monday, May 9, 2022
CONTACT: Adam Eidinger
Press@RightToTryPsilocybin.com | 202-744-2671
Terminally Ill Cancer Patient and Supporters of the Right to Try Act to Shut Down Drug Enforcement Administration Headquarters
The DEA Is Preventing Terminally-Ill Patients Their Legal Right to Try Psilocybin by Invoking the Controlled Substances Act
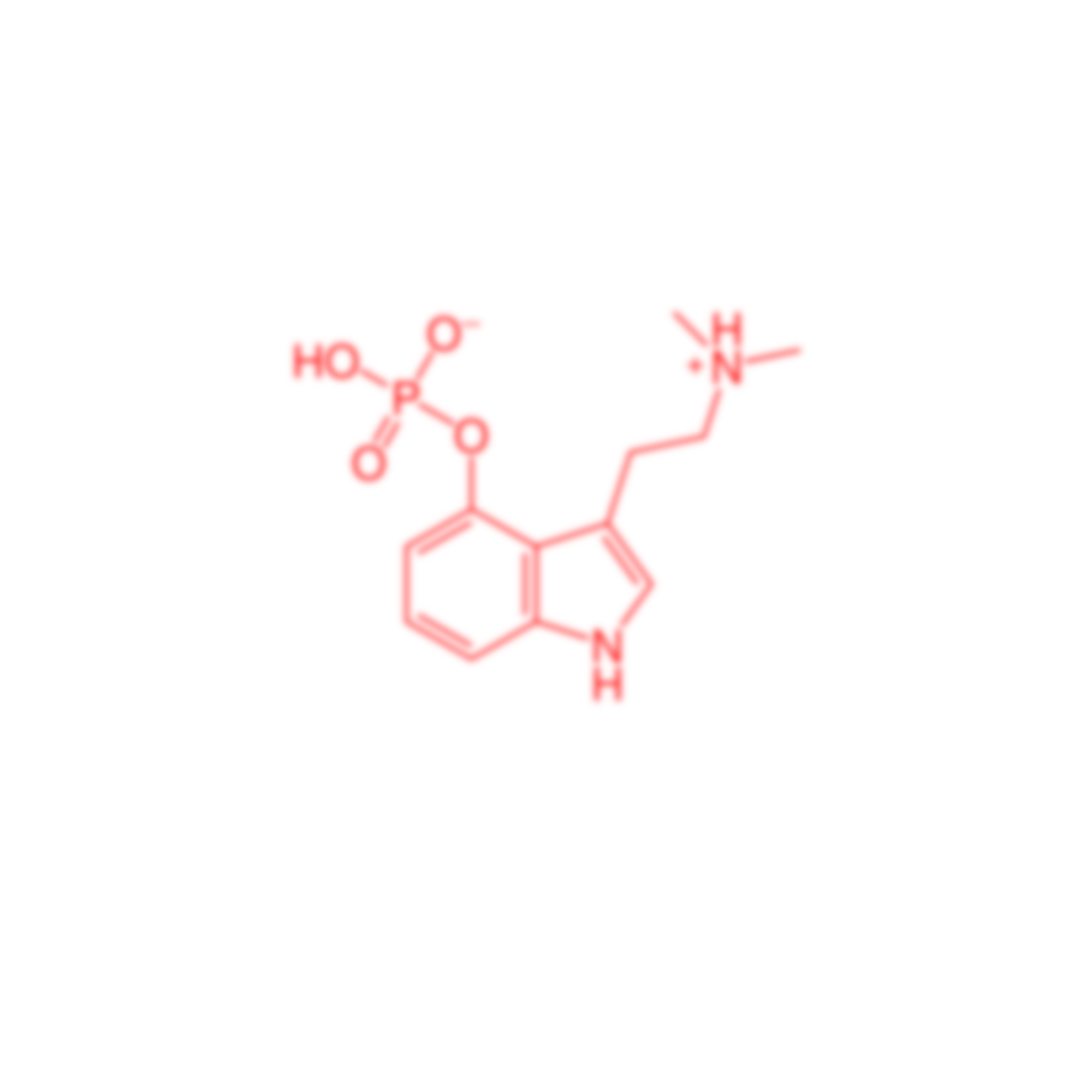
ARLINGTON, VA — On Monday, May, 9, at 12:00pm ET, supporters of U.S. citizens’ right to try psilocybin will hold a rally and engage in nonviolent civil disobedience at the Drug Enforcement Administration (DEA) headquarters. Passed by Congress and signed by President Trump in 2018, the Right to Try Act affords citizens with life-threatening conditions the right to use experimental drugs as long as the drug has completed Phase 1 clinical trials and remains under investigation. Laboratory-produced psilocybin, the psychoactive chemical commonly found in psilocybe mushrooms, has completed Phase 1 and Phase 2 trials, remains under investigation, and has been designated as a “breakthrough therapy” by the Federal Drug Administration. Psilocybin has been shown to bring ‘Immediate, substantial and sustained’ relief from anxiety and depression in dying patients. Unfortunately, the DEA has prevented terminally-ill patients from receiving the drug for nearly two years by incorrectly citing the Controlled Substances Act and psilocybin’s status as a Schedule I drug.
“My cancer is progressing and I am running out of time to access this promising medicine. It is not just the DEA’s refusal to grant access, but the fact that they have yet to confirm receipt and/or respond to our waiver request from early February is inhumane, unjust, and downright shameful. This agency is not above the law, and in this instance a law that was passed with overwhelming unilateral support from our legislators.” says Erinn Baldeschwiler, a mother of two teenagers, who was diagnosed with Stage 4 metastatic breast cancer in 2020.
Ms. Baldeschwiler’s doctor, Dr. Sunil Aggarwal, a Seattle palliative care physician and co-director of the Advanced Integrative Medical Science Institute, sought permission from the DEA to obtain psilocybin for his patients. As a Schedule I substance, this requires the DEA to sign off on his request, which it has refused to do. This began a legal battle that is still ongoing.
“We have patiently explored every avenue with DEA to gain its approval for terminally ill patients to access psilocybin for relief of debilitating anxiety and depression. Access is intended by duly enacted state and federal Right to Try (RTT) laws. Yet the DEA has engaged in delay and obstruction. This is unacceptable. My clients are running out of time. There is urgent need for the DEA to accommodate RTT and enable access. This demonstration shines the bright light of public concern and outrage on this agency’s conduct. Not one more day ought to go by without the DEA creating a path to access.” says Kathryn Tucker, Special Counsel with the Emerge Law Group and the Lead Counsel in the legal battle for the doctors and patients.
As their lawsuit has moved through the courts numerous members of Congress and Attorneys General have reached out to the DEA and submitted amicus briefs (See Timeline below). All appeals have fallen on deaf ears and have given reason for advocates to come to the DEA Headquarters to demand action.
“Psilocybin therapy has the power to help patients in the final months of their life, to break through fear and anxiety, and enjoy their family and loved ones” says David Bronner, the Cosmic Engagement Officer of Dr. Bronner’s, the nation’s leading organic and fair trade soap company, which has supported efforts to ease restrictions on psilocybin. “DEA is way out of line denying Americans access to this kind of relief in their last days.” Bronner will be speaking at the rally and engaging in nonviolent civil disobedience in support of the patients.
# # #